Disclaimer / Please be sure to read
Quantum Ecology is untested and unacknowledged by current physical science. Therefore, please note that we are not responsible for any problems that may arise from applying the theories and techniques on this site without our involvement. If you would like to add Quantum Ecology perspective to your current research, please contact us. We will work with you to achieve the best results.
No.7
Concept of atoms based on Quantum Ecology
Concept of atoms based on Quantum Ecology
This is a site that introduces you to the world of Quantum Ecology.
This time, we will review No. 3 to No. 6.
Let's take a look back at the explanations so far.
Topic No. 3 was, "What are atoms made of?"
I explained that each atom of any type of element is made of the same number of protons and electrons, and all atoms except hydrogen atoms have neutrons.
In No. 4, I explained that atoms are a collection of positively charged protons and negatively charged electrons and neutrons, but they do not carry any electric charge. I will explain this again.
Atoms are collections of positive (protons) and negative (electrons) electricity. However, every atom has the same number of protons and electrons, and the same number of positive and negative electricity, so the electricity is ±0, and the atom is not charged with electricity.
I explained that normally you don't need to take neutrons into account because electricity is ±0 and the only effect is magnetic force.
In No. 5, I explained the definition of quantum according to Quantum Ecology.
Atoms are made up of three types of energy: protons with positive electricity, electrons with negative electricity, and neutrons with magnetic force, and in Quantum Ecology the word "quantum" refers to these three.
I then explained that when the number of quanta that make up a substance changes, the substance changes into a different substance.
No.6 was about when atoms become electric. I explained that when electrons move out of an atom, the atom becomes electric.
Let's review this.
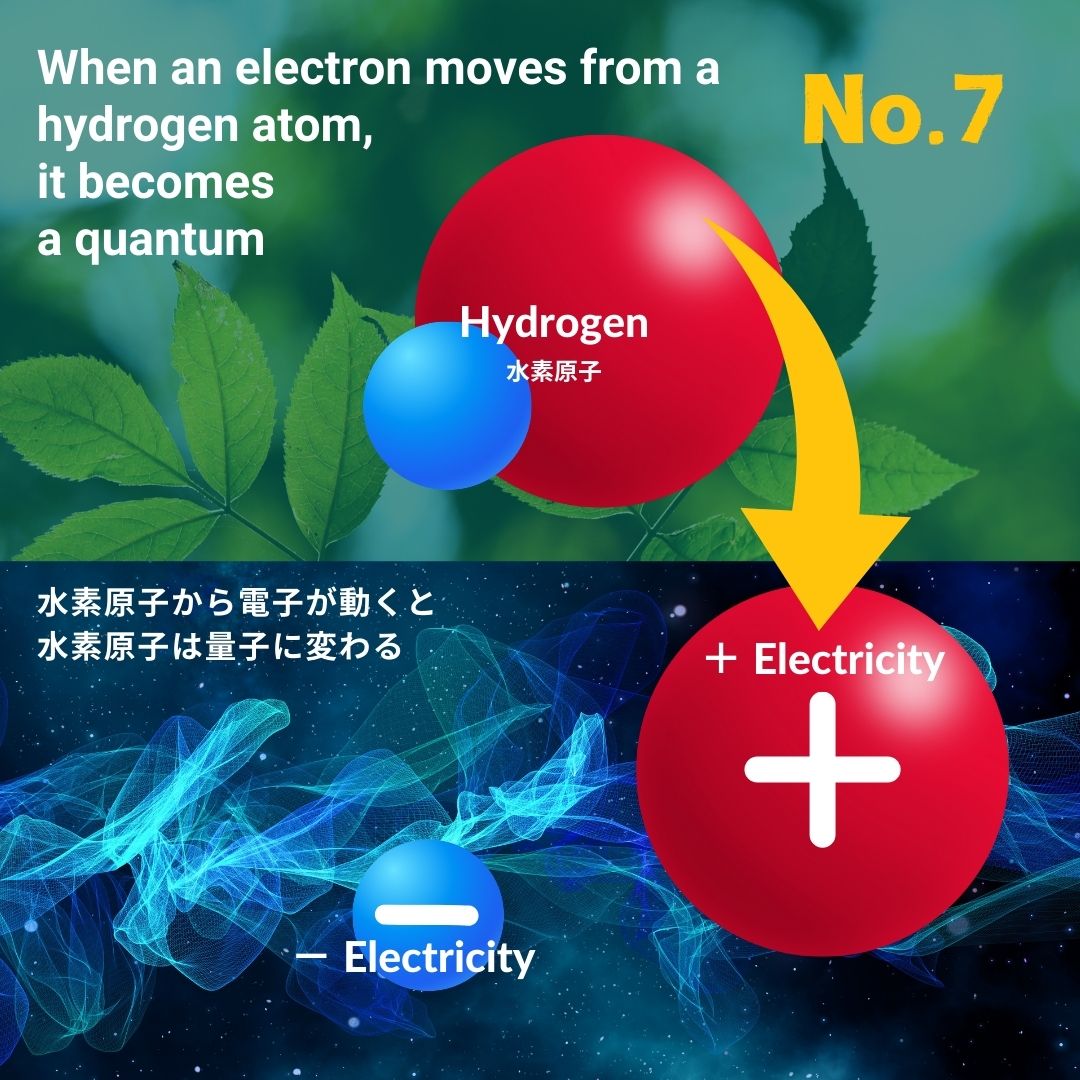
I will explain once again the state in which a hydrogen atom becomes electrically charged.
A hydrogen atom is made up of one positive charge called a proton and one negative charge called an electron.
Hydrogen atoms are made up of +1 and -1 charges, but the total charge is ±0, so hydrogen atoms are not electrically charged.
However, when an electron moves from this hydrogen atom, the hydrogen atom becomes electrically charged.
A hydrogen atom has one electron, and sometimes this one electron moves and goes somewhere. When this happens, the hydrogen atom becomes just one proton, and has an electrical charge of +1.
In other words, this hydrogen atom changed from being a "±0 substance with no electric charge" to a "+1 quantum called a proton."
From the perspective of Quantum Ecology, this means that "electrons have moved from the hydrogen atom, and the number of quanta in the hydrogen atom has changed.''
Let me explain No. 5 again.
From the perspective of Quantum Ecology, "quantum changes into a different substance when the number of quanta changes."
You can understand that when an electron moves from a hydrogen atom, the number of quanta clearly changes.
In other words, according to Quantum Ecology, when an electron moves and only a proton remains, we must think of the hydrogen atom as "will change into something different because the number of quanta has changed."
When an electron moves from a hydrogen atom, the hydrogen atom changes from an "object" with an electrical potential of ±0 to an electrical potential of +1 due to one proton, becoming a "quantum."
At the same time, the electron that moved from the hydrogen atom is somewhere, wandering around as a "quantum" of a single electron with an energy of -1.
These will eventually become components of different things than the things they were making together before electrons started moving, creating different things.
By applying Quantum Ecology in this way, we can understand that the phenomenon occurs when "quantum become something different when their numbers change."
What is important here is how a single proton or a single wandering electron affects its surroundings.
Quantum Ecology believes that unless we understand atoms in this way, we will not be able to properly understand the phenomena of the natural world.
What I want you to remember today is this:
When an electron moves from a ±0 hydrogen atom, the hydrogen atom changes to +1.
This is the state in which the hydrogen atom has changed from a "±0 substance" to a "+1 quantum."
In other words, this hydrogen atom will change into something different because the number of quanta has changed.

